AseptiKits is now registered in the SAM system and available for purchase by ALL government agencies.
- SAM: C7H1DESNVMN4
- CAGE: 9N8Z0
- NAICS: 423460
- POC: Becky Thorne
VP of Sales and Marketing
- Phone: 844-863-5170 X4828
- Email: becky@aseptikits.com
AseptiKits mission is to make serum tears affordable and convenient for all patients who need them.
About AseptiKits, LLC
Founded in 2019, AseptiKits, LLC is a small, privately held, medical device company that researches, develops, patents, manufactures, and sells new products based on patented technologies.
Our organization partners have over 150 years of combined experience in the medical device and pharmacy world. This enables a vast depth of understanding of FDA regulations, standards relative to USP, medical devices, quality assurance and good manufacturing practices.
Currently working within the private sector, AseptiKits has well over 100 labs, phlebotomists and eye doctors in 32 states who regularly purchase PALA kits to process serum tears for patients who suffer from dry eyes and other ocular conditions. Customers include doctors referring paitents to labs and phlebotomists, and doctors who process the serum tears within their own offices.
Key Accounts
ARCpoint Labs
National chain - currently located in 30 states with over 75 sites offering serum tears onsite processing to hundreds of patients every month.
-
Court Wilkins, OD
Utah Eye Centers (4 locations), 16 practitioners offering serum tears to patients with dry eyes. - College of Optometry at Rocky Mountain University of Health Professionals. Dean of Clinical Affairs.
-
Riverwood Eye Center:
Provo, Utah, offering serum tears for patients with dry eyes and other ocular issues. - PALA kits also used by Dr. Ward in Africa (eSwatini) as he works in conjunction with the Luke Foundation.

Key Differentiators
Decreases costs for serum tears up to 50% compared to other services
Onsite patient blood draw and serum tears delivery within about an hour
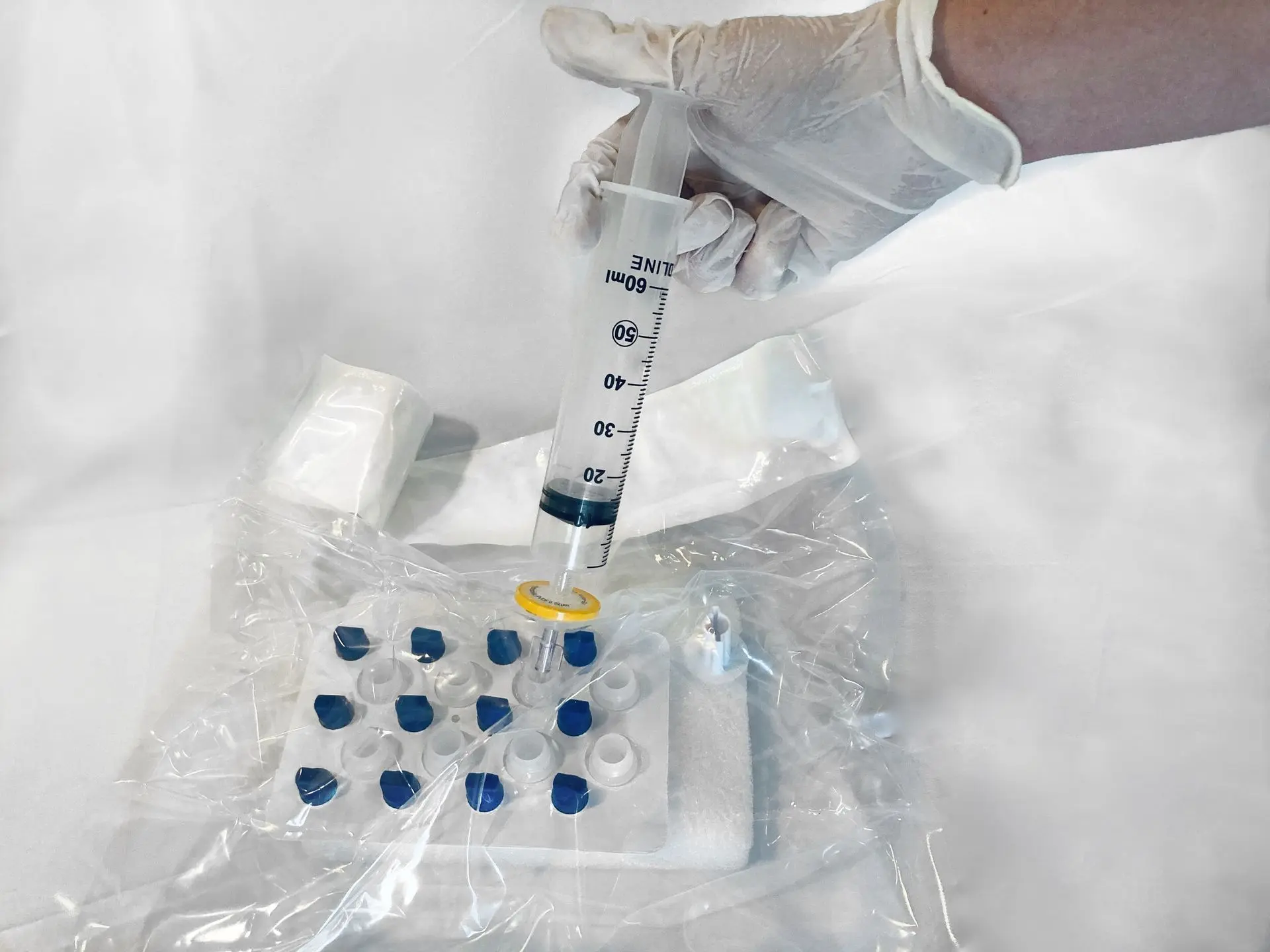
Clean room in a bag
Meets or exceeds FDA regulations and all USP Chapter 797 requirements for sterile preparations
Enables sterile preparations any place / anywhere
Single use, sterile preparation environment
Integrated 0.2 micron filtering into the sterile chamber
Containers capped and sealed prior to access
Closed system safety
Aseptic technique independent
40 mL, 60 mL, and 70 mL kits
Contact us:
- Sales@AseptiKits.com
- +1 844-863-5170
Our friendly team is here to help with questions, purchases, or concerns.
Send an email: